
What Is Ultra Pure Water? Understanding Its Benefits and Uses
A UNEP study of 75,000 water bodies around the world established that 40% are polluted. From consumption to industrial processes, water plays a
Give us a Call
Call for Emergency Services
Compliant water is key in a successful hemodialysis treatment center. The patients’ 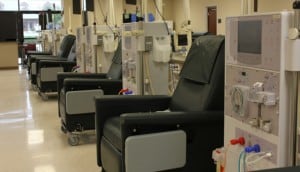 health is of utmost importance and as time goes on required standards are ever increasing and at times can be a challenge for those in the field to achieve. Most water treatment companies shy away from the requirements of the Association for the Advancement of Medical Instrumentation (AAMI) and Center for Medicaid and Medicare Services-(CMS) Guidelines, leaving a number of hospitals and treatment centers searching for a reliable partner in satisfying the needs of the new standards. The requirements are getting stricter and will likely continue to be more stringent. With wide reaching changes being published in 2008 turning a number of centers on their heads, an even bigger wake- up call occurred in 2011, when further reaching standards were published. Although still lower in some areas than some foreign standards, such as found in the United Kingdom, they continue to strive for near perfection and the standards are likely to only increase. Concerns hitting the bacteria levels, now requiring a 7 day incubation period, can be very real. More than the minimum monthly sanitization requirement can be a necessity on older and complex loop systems resulting in more down time and costly chemical sanitizations to be compliant. Issues filtering out chloramines versus chlorine when the municipality or primary disinfection system shifts gears can leave you scrambling trying to remove the more persistent chloramines. When endotoxins are elevated above the more strict action level, there will be more of a focus on your water treatment system design than perhaps in the past. Being prepared to combat these issues before they start begins with studying your current system and looking for proactive solutions and a partnership with a reliable and knowledgeable supplier.
health is of utmost importance and as time goes on required standards are ever increasing and at times can be a challenge for those in the field to achieve. Most water treatment companies shy away from the requirements of the Association for the Advancement of Medical Instrumentation (AAMI) and Center for Medicaid and Medicare Services-(CMS) Guidelines, leaving a number of hospitals and treatment centers searching for a reliable partner in satisfying the needs of the new standards. The requirements are getting stricter and will likely continue to be more stringent. With wide reaching changes being published in 2008 turning a number of centers on their heads, an even bigger wake- up call occurred in 2011, when further reaching standards were published. Although still lower in some areas than some foreign standards, such as found in the United Kingdom, they continue to strive for near perfection and the standards are likely to only increase. Concerns hitting the bacteria levels, now requiring a 7 day incubation period, can be very real. More than the minimum monthly sanitization requirement can be a necessity on older and complex loop systems resulting in more down time and costly chemical sanitizations to be compliant. Issues filtering out chloramines versus chlorine when the municipality or primary disinfection system shifts gears can leave you scrambling trying to remove the more persistent chloramines. When endotoxins are elevated above the more strict action level, there will be more of a focus on your water treatment system design than perhaps in the past. Being prepared to combat these issues before they start begins with studying your current system and looking for proactive solutions and a partnership with a reliable and knowledgeable supplier.
What new technologies are out there to help reach the ever increasing requirements? What are some good general guidelines those in the field of hemodialysis should consider?
Heat Sanitization: Heat sanitizable Central Reverse Osmosis systems and Portable Reverse Osmosis systems are now on the scene. Loop sanitization systems are now 510K FDA certification approved which can greatly reduce the expense associated with required monthly loop sanitizations and can allow for more frequent sanitization of the system to keep numbers at or below required specifications.
Ozone Sanitization: Ozone is now 510K approved by the FDA for loop sanitizations. Ozone FDA Press Release This application should be carefully considered but can be the right prescription. The dissipation of the Ozone is helpful in getting a system back on-line quickly but the highly effective kill rates can help you destroy any potential loop bio-film on an older loop system or prevent future concerns.
Sizing Systems Properly: Selecting new equipment that can be of a more modular design is key. Although it is important to always document all items of a system and be 510K compliant, looking at a system for the long-term and not over or under-sizing can greatly affect the ability of a team to achieve the CMS/ISO/AAMI recommendations and regulations and save costly complete system revamps. Proper return flow rates are key in meeting endotoxin and bacteria levels. Storage should be minimized as much as possible since stored water degrades over time, and can lead to bacterial growth.
Locating Your Water Treatment Equipment as close to the unit as possible:This AAMI recommendation is there for a reason. Not only do shorter loops help prevent endotoxin and bacterial concerns, equipment that is closer to the actual unit helps ensure readings are taken timely and any potential issues with a water system are noticed right away.
Make Sure People on Staff are Thoroughly Trained on the Water Treatment System: Regardless of what company you may partner with to maintain your system, they are not there 24/7 like your staff is. Taking required and proper readings by the staff on site is key. Ensuring that all components of the system are working as designed is important as well.
Follow Equipment Manufacturer’s Guidelines: Spending a little more on maintenance and perhaps a preventative maintenance contract with a trained factory certified equipment representative can help eliminate not only down time in your unit but offers external validity to your operation. Additionally, it ensures that you have someone available if there is a system failure by someone who knows your system, and its needs and requirements. Merely assuming that your staff is properly trained and following guidelines or ensuring the system is functioning properly can be a costly mistake.
Self-Audit: Consider a self-audit. Do an informal in-house audit to make sure all CMS/AAMI best practices and guidelines are being followed. If there are issues, revisit your internal procedures and make corrections immediately to ensure ongoing compliance.
Documentation: Properly labeling of system components and a proper and complete diagram on site are often a large focal point of an inspection. If system changes were made from the original design, be sure to label all items properly, update on site documentation and follow all FDA 510K requirements for the occurrence. Any alterations to a loop system or a registered medical device require certain steps that must be followed closely to remain in compliance. Change control records and Quality System Regulations must be followed.
For feedback and an analysis of your current central water treatment system or to obtain more information on the new water treatment for hemodialysis trends and product offerings please contact us at Absolute Water Technologies. www.absolutewatertech.com 1-866-986-6860.

A UNEP study of 75,000 water bodies around the world established that 40% are polluted. From consumption to industrial processes, water plays a

Water quality is crucial for safety in medical and pharmaceutical environments. With over 2 billion people facing unsafe water conditions globally, access

The water used in hospitals, pharmaceuticals, and labs is far from everyday drinking water. It needs to be purified to meet safety
Absolute Water Technologies
(866) 986-6860
info@absolutewatertech.com
Absolute Water Technologies understands that your privacy is extremely important to you. We understand, it is just as much a concern for us.
Absolute Water Technologies is in the business of selling high-quality equipment for your water purification needs. Not your personal or business information.
Whenever you purchase from our online store, any and all credit card transactions are handled through our gateway processors. We currently offer PayPal for our customers who want to use their PayPal account or credit card. In addition, we also offer straight credit card processing through Authorize.net. In both cases, the respective Privacy Policies for each solution is available at the following websites. Please be certain to review this information before ordering through our online store.
Absolute Water Technologies does not store any financial information of any of our customers on this site or domain.
© All rights reserved Sitemap Privacy Policy Terms of Use